Going the distance - and why it matters in gait analysis
A footprint, that is. With CatWalk XT, you can extract a lot of information from just one footprint. In this post, I am taking it a step further by talking about the relationship between prints.
Posted by
Published on
Thu 28 Jun. 2012
Topics
| Arthritis | Ataxia | CatWalk XT | Foot Prints | Gait | Gait Analysis | Mice | Rats | Spinal Cord Injury |
Parameters that describe the relation and distances between footfalls
Last week I wrote about the value of a print. A footprint, that is. With CatWalk XT, you can extract a lot of information from just one footprint. In this post, I am taking it a step further by talking about the relationship between prints.
In the study of many different disorders that affect the nervous system, muscles, or bones, it is important to know how the animal walks. Does it have a regular gait, following a normal pattern of footsteps? Or can we detect a lack of coordination, or ataxia? Those are important behavioral observations in many studies. So how can you detect them: by looking at the relation between prints.
Clinicians are trained to visually observe the gait of their patients to detect abnormalities. They can ask their patients to walk a certain way or direction, to watch their stability and balance. But what if your patient is a small rodent?
Small rodents are often used in research on a wide scale of disorders that cause abnormalities in gait. Automated gait analysis allows for accurate assessment of several parameters (behavioral endpoints) when you can’t ask your animal to please walk in a straight line.
Regularity
In many disease models, the regularity with which the animal walks is important. For example, animal models of ataxia [1], arthritis [5,9], stroke [4], and spinal cord injury [2,3,7] have a lower regularity.
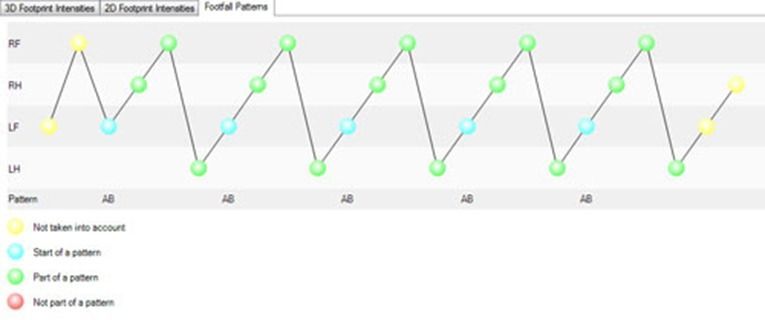
So how can you detect irregularity automatically? By using gait analysis software that recognizes ‘normal’ footfall patterns, and detects deviations from this pattern.
Solutions such as CatWalk XT recognizes several normal gait patterns, including: left front, right front, left hind, right hind. This pattern is described in literature as cruciate. If during the run, the animal places one of its paws outside the pattern, that paw is labeled as “not part of a pattern”. The number of irregular paw placements relative to the regular paw placements is represented in the regularity index.
Base of support
The base of support is the distance between either the two hind paws or the two front paws. In spinal cord injury, animals seem to have trouble keeping balance, causing them to place their hind paws wider apart than normal [3,6]. Tail and abdomen drags are not uncommon [3]. In contrast, Parkinson’s models are reported to have a smaller base of support in their front paws [10], placing them closer together than non-affected controls. This is similar to the posture human Parkinson’s patients show; they also keep their feet closer together and show small movements with a hunched over posture.
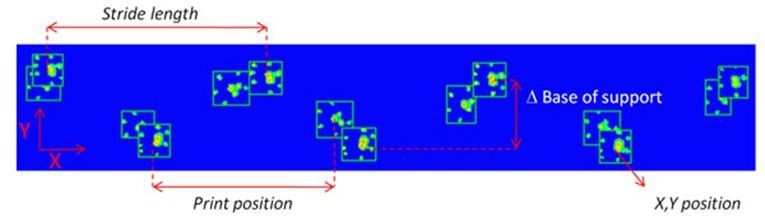
Stride length
Stride length is the distance between the successive placement of the same paw - in other words, the distance one paw travels during one step. Again, similar to human patients, Parkinson’s model animals are reported to take smaller steps [10], which can be confirmed with smaller stride lengths found with CatWalk XT automatic gait analysis. There are also reports of a decreased stride length in ataxic animals [1] and spinal cord injury models [3,8].
Curious to find out what more you can measure with automated gait analysis? Sign up to be one of the first to read the last blog in this series!
Other blogs in this series
Other blogs in this series are now online:
- What gait can tell: 3 blogs that will help you understand
- What a print can tell
- Time is of the essence - how you can use it in gait analysis
Or download the free white paper!
Of course, you can always go to www.noldus.com/catwalk-xt for more information on automated gait analysis.
References
- Cendelín, J.; Voller, J.; Vožeh, F. (2010). Ataxic gait analysis in a mouse model of the olivocerebellar degeneration. Behavioural Brain Research, 210, 8-15.
- Galvan, M.D.; Luchetti, S.; Burgos, A.M.; Nguyen, H.X.; Hooshmand, M.J.; Hamers, F.P.T.; Anderson, A.J. (2008). Deficiency in complement C1q improves histological and functional locomotor outcome after spinal cord injury. The Journal of Neuroscience, 28(51), 13876-13888.
- Hamers, F.P.T.; Lankhorst, A.J.; Van Laar, T.J.; Veldhuis, W.B.; Gispen, W.H. (2001). Automated quantitative gait analysis during overground locomotion in the rat: its application to spinal cord contusion and transection injuries. Journal of Neurotrauma, 18, 187-201.
- Hetze, S.; Römer, C.; Teufelhart, C.; Meisel, A.; Engel, O. (2012). Gait analysis as a method for assessing neurological outcome in a mouse model of stroke. Journal of Neuroscience Methods, 206, 7-14.
- Hoffmann, M.H.; Hopf, R.; Niederreiter, B.; Redl, H.; Smolen, J.S.; Steiner, G. (2010). Gait changes precede overt arthritis and strongly correlate with symptoms and histopathological events in pristane-induced arthritis. Arthritis Research & Therapy, 12, R41.
- Kloos, A.D.; Fisher, L.C.; Detloff, M.R.; Hassenzahl, D.L.; Basso, D.M. (2005). Stepwise motor and all-or-none sensory recovery is associated with nonlinear sparing after incremental spinal cord injury in rats. Experimental Neurology, 191, 251-265.
- Koopmans, G.C.; Deumens, R.; Buss, A.; Geoghegan, L.; Myint, A.M.; Honig, W.H.H.; Kern, N.; Joosten, E.A.; Noth, J.; Brook, G.A. (2009). Acute rolipram/thalidomide treatment improves tissue sparing and locomotion after experimental spinal cord injury. Experimental Neurology, 216, 490-498.
- Lee, H.; McKeon, R.J.; Bellamkonda, R.V. (2010). Sustained delivery of thermostabilized chABC enhance axonal sprouting and functional recovery after spinal cord injury. PNAS, 107(8), 3340-3345.
- Möller, K.Ä.; Berge, O.-G.; Hamers, F.P.T. (2008). Using the CatWalk method to assess weight-baring and pain behaviour in walking rats with ankle joint monoarthritis induces by carrageenan: effects of morphine and rofecoxib. Journal of Neuroscience Methods, 174, 1-9.
- Westin, J.E.; Janssen, M.L.F.; Sager, T.N.; Temel, Y. (2012). Automatic gait analysis in bilateral Parkinsonian rats and the role of L-DOPA therapy. Behavioural Brain Research, 226, 519-528.
Related Posts

Testing without stress: high-throughput phenotyping
Gait analysis after a bone fracture

