A to Z on optogenetics and video tracking studies
Optogenetics allow researchers to either activate or inhibit neurons in the brain. To see how this affects behavior, you need to record that behavior and synchronize it with the stimulation. Here's how.
Posted by
Published on
Thu 08 Oct. 2020
Topics
| EthoVision XT | Optogenetics | PhenoTyper |
Today, I’ve snuck into the blog from the Noldus Support and training department to talk about EthoVision XT. One of the things that keeps my job interesting is that scientists always want to try something new. Existing tools get applied to new problems in new ways. Optogenetics provides a great example.
Optogenetics
Optogenetics is a transformative technique which allows researchers to either activate or inhibit neurons in the brain, in order to determine the function of those brain cells. This technique is applied in living fruit flies, nematodes, zebrafish, and mice and rats.
The technique of optogenetics
In an optogenetics experiment, neurons are treated in various ways to incorporate light sensitive receptor proteins in their cell membranes. A fiber optic cable is implanted in the animal to bring light to the targeted cells. (In case of developing zebrafish, for example, no implants are necessary as larvae are transparent.) When these cells are exposed to light of the correct wavelength, ion channels open, causing the neuron to fire.
These channels remain open as long as the light remains on, preventing the neuron from returning to its resting state. The result is that the neurons are “turned off” or inhibited; whatever signals they would normally respond to now elicit no response.

Activating neurons
Alternatively, the researcher may want to see what happens when the neurons are activated. In this case the light is flashed rapidly (typically on the order of 20 times per second). When the light turns on, the neuron fires, and when it turns off, the neuron recovers so that it is ready to fire again. Flashing the light repeatedly causes the neuron (or neurons) to fire rapidly and repeatedly.
Optogenetics in video tracking experiments
If the goal is to see how these changes affect behavior, then you need a tool to record that behavior and synchronize it with the stimulation. This is where EthoVision XT comes into the picture.
An optogenetics system consists of EthoVision XT, a computer, a USB IO-box, a light source with a trigger input, and usually a pulse generator. The light source may be a laser or a high intensity LED. In addition, fiber optics, connectors, and a canula get the light into the animal’s brain.
Inhibiting neurons during a behavioral test
Inhibition is the easiest scenario, since the light simply needs to be turned on and remain on as long as inhibition should continue. At the appropriate point during the trial, EthoVision XT uses the USB IO-box to trigger the light source. The light remains on until EthoVision turns off the signal.
Using pulse generators to activate neurons
Activating the neurons requires one additional step. It’s not enough to turn the light on and leave it on, it must also be rapidly flashed on and off repeatedly. This is where a pulse generator must be added. EthoVision XT triggers the pulse generator to start, and the pulse generator handles the rapid pulsing of the light source.

Although we often use the Pulser by Prizmatix (shown in the picture with PhenoTyper setup above), EthoVision XT is highly adaptable and can be used with a wide variety of pulse generators. This type of equipment normally uses a simple 5V trigger signal, regardless of maker, so it’s just a matter of providing the correct type of cable and setting the pulse generator correctly. An Arduino can also be used in place of a pulse generator for those willing to do their own wiring.
Controlling stimulation
Now that the hardware is set up, when should the light be turned on? In EthoVision XT, we use the Trial Control to define how long the trial should run, and at what points devices should be turned on and off. The simplest possible optogenetics trial would present the stimulation (or inhibition) at a particular time.
The Trial Control is simply a flow chart of the events of the trial. Individual steps in the trial can be named to make it easier to refer to them in the analysis. Here, we have an initial 5 minute pre-stimulation period, followed by a minute of stimulation, and then 5 minutes post-stimulation:

Any number of events can be strung together this way. The events flow in the direction of the arrows, and when they reach “Stop Track”, the trial is over.
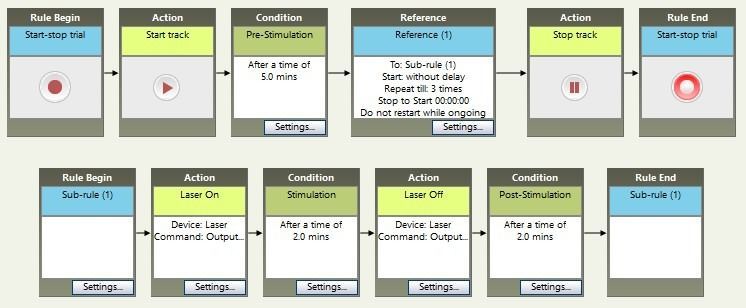
Repeated stimulation
However, sometimes you want to repeat events over and over. It’s possible to do that by stringing many boxes together, but it can be easier to create a Sub-Rule which repeats a predefined number of times.
In this example, after the 5 minute pre-stimulation period, the sub-rule is called three times. Each time, the stimulation is activated for two minutes, then turned off for two minutes. When the sub-rule has completed the third run-through, we continue to the Stop Track box and the trial ends.
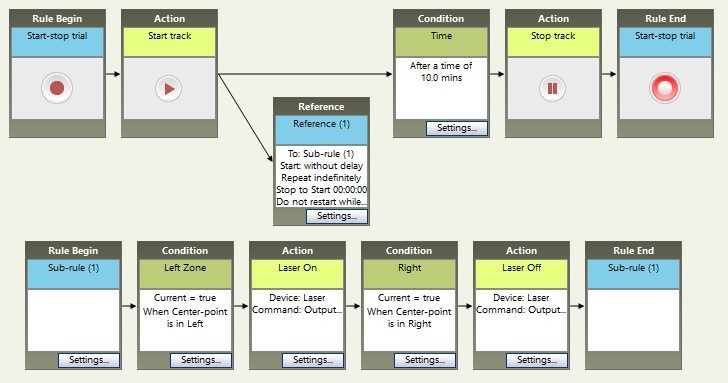
Place-preference
A common scenario is a place preference test. In this case, the stimulation (or inhibition) occurs whenever the animal is in a particular zone. Instead of running the sub-rule a pre-determined number of times, we want it to run repeatedly, checking whether the animal is in the zone or not. In this example, the stimulation begins when the animal enters the Left zone, and stops when the animal moves to the Right zone. Because the sub-rule will never complete (it will just keep checking which zone the animal is in), we add a time condition to control the duration of the whole trial (10 minutes in this example).
Other options
Although in this example we used the zone to trigger the stimulation, in fact it’s possible to use any behavior the same way. Stimulation (or, for that matter, lights, sounds, pellet dispensers, or any other type of device) can be triggered based on the activity level, velocity, degree of turning, or other behavioral measures. It could also be based on sensors, for example when a lever is pressed or a nose-poke sensor is triggered. Whatever the needs of your research, EthoVision XT is ready.
Read more about optogenetics on our website and in this blog post.
https://noldus.com/applications/optogenetics
https://noldus.com/blog/optogenetics
https://noldus.com/blog/optogenetics-and-operant-conditioning
Related Posts

Motivation and eating: deep brain imaging in freely moving mice

No science fiction: Magnetogenetics and how to induce animal behavior

