September 26, 2024
Noldus partners with Zhejiang Institute of Medical Device Testing
This month, the Medical Device Usability Engineering Laboratory of Zhejiang Institute of Medical Device Testing (MDST) in Hangzhou was officially opened. The opening ceremony and seminar were held at the Linping Economic and Technological Development Zone in Hangzhou. Noldus is proud to contribute to this initiative.


Image source : MDST
Medical technology in China
China is keen to boost its production of medical technology to reduce its reliance on imports. In 2015, the Chinese government launched its "Made in China 2025" policy to increase the proportion of domestically made mid- to high-end medical devices used in Chinese hospitals to 70% by 2025, and to 95% by 2030 (Ong, 2024). In order to safeguard the quality and safety of novel medical equipment, medical device inspection laboratories are being established across the country. Among the aspects of medical devices being tested are human factors design and usability of hardware and software.
About the laboratory
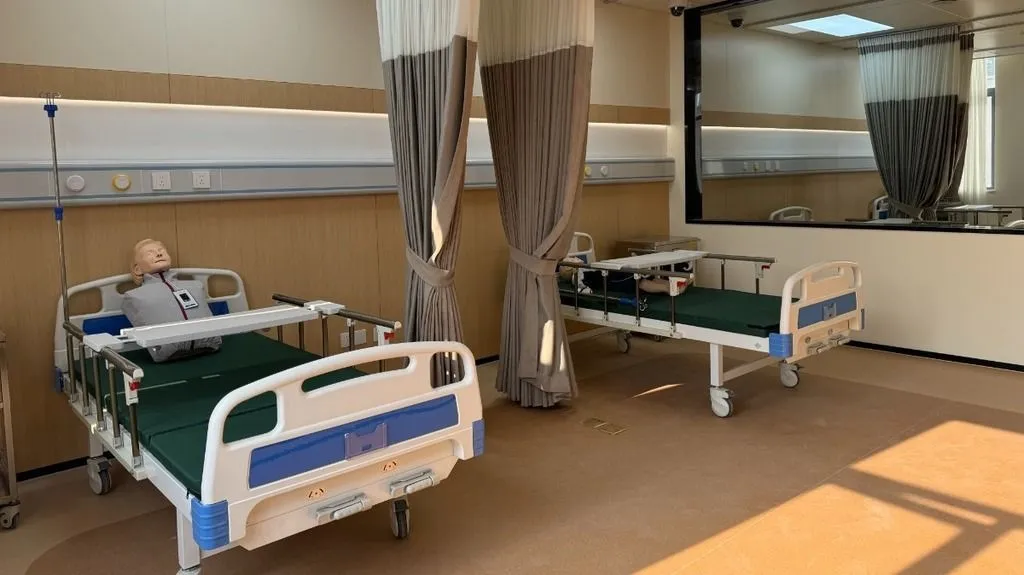
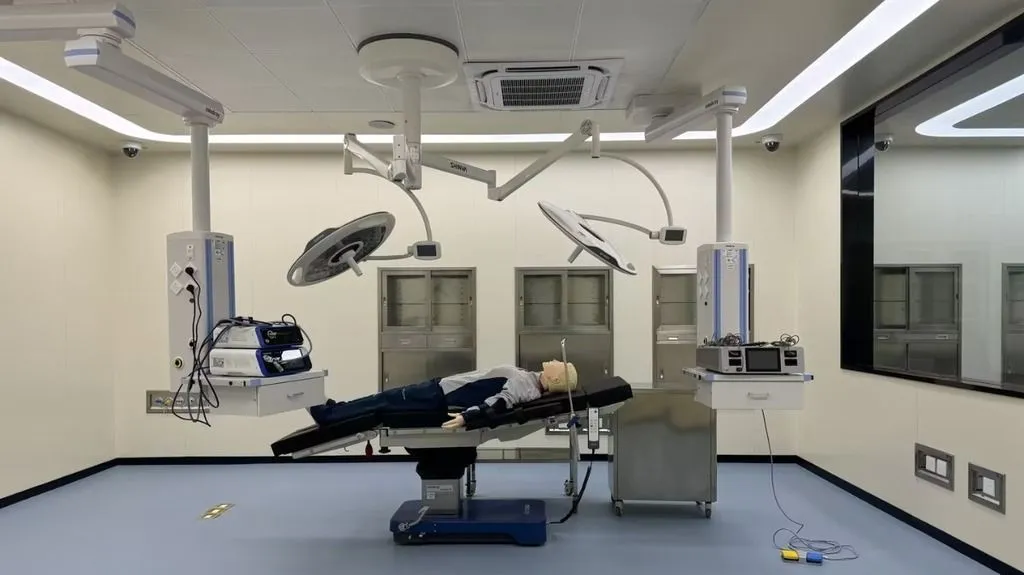
The Medical Device Usability Engineering lab (MDST) features multiple highly advanced simulation and testing environments. These include operating rooms, ICUs, double rooms, nurses’ stations, examination rooms, and simulated home environments. The state-of-the-art lab is equipped with advanced software and hardware, such as medical simulators, single-sided observation mirrors, behavior analysis systems (The Observer XT), multi-video recording software (MediaRecorder), and video recording and playback systems (Viso).
Laboratory development
The lab is being built in phases, with space reserved for future expansion. This allows for early implementation of usability testing, maximizing investment efficiency. Once complete, the lab will handle testing for two types of medical devices simultaneously.
Guidelines Medical Device Usability Engineering
The National Medical Products Administration (NMPA) released the “Guidelines for Registration and Review of Medical Device Usability Engineering” on March 19, 2024. These guidelines, effective from October 8, 2024, aim to standardize usability management. The new lab marks significant progress in this field, promoting usability evaluation and optimization, improving product safety and user experience, and meeting clinical needs.
To enhance the lab further, the Zhejiang Institute will introduce advanced equipment like eye trackers and finger tracking systems.
Noldus will also continue to assist the Zhejiang Institute of Medical Device Testing in completing the next phase of work and plans for the Medical Device Usability Engineering Laboratory, by providing the highest quality research solutions and consulting services.