The relationship between social hierarchy and social stress
Today, Deepika Patel kindly shares her research on social structures with us. She investigated rat behavior in detail, using several test paradigms.
Posted by
Published on
Wed 14 Oct. 2020
Topics
| EthoVision XT | Stress | Coding Behavior | Rats | Social Behavior Research | Social Hierarchy | Social Interaction | Video Tracking |

Today, Deepika Patel kindly shares her research on social structures with us. During her PhD at GELIFES (University of Groningen), she investigated rat behavior in detail, using several test paradigms. Congratulations with your thesis, Deepika!
A key feature of group living is the establishment of dominance relationships in competition for resources. The competition for a high rank is often accompanied with social conflicts due to a natural result of interactions between individuals, which can be a source of chronic stress leading to stress-related psychopathologies.
Social structures
Animals live together in groups with a hierarchical structure installed with dominant, subdominant and subordinate males. According to various studies this can induce chronic social stress of subordination [1].
The social dominance structure in humans is more complicated compared to the animal kingdom. Looking at the socioeconomic status (SES) however, the stepwise descent predicts increased risks of cardiovascular diseases, strokes and psychiatric diseases [2,3].
Dominance studies in animal models
Measuring the true consequences of a dominance ranking in humans is rather difficult since we live in multiple social networks simultaneously [4]. Therefore, studies in animal models, on the consequences of high or low dominance ranking in social structures, are of importance in understanding the effect of hierarchical status on our well-being.
Hence, we investigated the consequences of different hierarchical rankings on brain and behavior in wild-type Groningen (WTG) rats living in colony-like structure. Specifically, we focused on behavioral, physiological and neurobiological indicators of chronic social stress exposure during colony housing.

Using wild-derived rats
Most rodents are social animals by nature, living together in hierarchical colonies. There are several well-characterized tools used to measure social dominance in rodents [5]. For instance, agonistic behavior is often used as a marker for the determination of dominance.
In our experiments, we have used feral rats of which the ancestors were caught in the wild and outbred for many generations in our laboratory. They specifically appear to have a rich and diverse behavioral repertoire, particularly in the social domain, and also display individual variability in offensiveness [6].
Establishment of social hierarchy among WTG rats
For the installation of social hierarchies among male WTG rats, male and female rats were co- housed in a colony-like structure called the Visible Burrow System (VBS) for 3 weeks. The VBS is a habitat containing continuously dark burrows and an open area with a circadian light regime.
Monitoring and recording
The VBS’ were monitored 24/7 using digital monochrome GigE cameras connected to a computer running MediaRecorder by Noldus. Formation and maintenance of hierarchy during the colony period was assessed by scoring agonistic behaviors using The Observer XT by Noldus. Male dominance was validated by scoring agonistic behavior towards an unfamiliar male intruder.
Behavioral observation manifested a clear formation of social hierarchy in male WTG rats. Dominants exhibited offensive behavior towards subordinate and intruder male rats, whereas subordinate males showed defensive and social avoidance behavior.
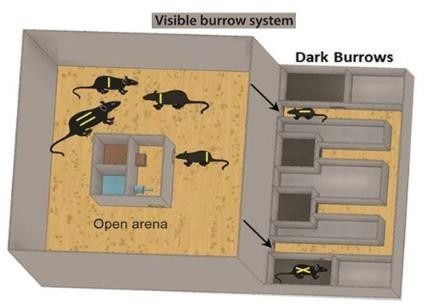
Schematic representation of the Visible Burrow System taken from [7].
Consequences of living in the colony-like structure
The first indication of stress vulnerability can be the change in an individual’s behavior [8]. Therefore, we behaviorally tested VBS housed male rats on social avoidance, open field and elevated plus maze tests for anxiety-like behaviors.
The behaviors shown were analyzed using the EthoVision XT by Noldus. Data from these tests did not show any significant differences among control, subordinate and dominant individuals. However, we did observe that the subordinate individuals spent maximum time in the dark burrows, hiding from the dominant rats.
Physiological and Neuronal changes
The amount of social stress that animals undergo in VBS housing is reflected in various physiological and neuronal alterations. Both dominants as well as subordinates lost a considerable amount of body weight and fat mass whereas adrenal glands were larger in VBS housed rats.
Furthermore, we studied proteins (p-cofilin/cofilin proteins ratio) involved in dendritic spine remodeling in several brain regions in dominant and subordinate males. A higher protein ratio was found in the subordinate as well as in dominant rats particularly in the medial prefrontal cortex and amygdala regions, respectively. This relates to enhanced behavioral flexibility in the subordinates and enhanced amygdala-dependent aggressive behavior in dominant males.
Visible burrow system as a model of chronic social stress
Although animals seem more stressed socially, chronic social stress induced by the VBS did not seem to affect general anxiety in male WTG rats. WTG rats appear to be highly resilient to the negative consequences of social stress, behaviorally, despite strong physiological and neurobiological response to social stress.
We speculate that to observe a differential behavioral response to stress in WTG rats, we might need more sensitive behavioral paradigms. Future studies using VBS could help in studying individual differences to stress susceptibility by using social status as a factor of vulnerability.
Image taken from PhD thesis- Deepika Patel
Extrapolating results to human life
Some of the common and consistent features experienced by conspecific individuals living in social groups are bullying, domestic violence and subordination. These are successfully modelled in the laboratory settings and have helped to investigate behavioral, neural and endocrine correlates of social stress.
Lastly, housing rats in semi-natural environments produces large individual differences in ranking position, making this a promising model to study underlying mechanisms of individual differences in social ranking and the potential role of high or low ranking in health and disease.
References
- Sapolsky, R.M. (2005). The influence of social hierarchy on primate health. Science (80), 308, 648–652, doi:10.1126/science.1106477.
- Cohen, S.; Doyle, W.J.; Baum, A. (2006). Socioeconomic Status Is Associated With Stress Hormones. Psychosom. Med., 68, 414–420, doi:10.1097/01.psy.0000221236.37158.b9.
- Goodman, E.; Slap, G.B.; Huang, B. (2003). The public health impact of socioeconomic status on adolescent depression and obesity. Am. J. Public Health., 93, 1844–50.
- Cummins, D.D. (2000). How the Social Environment Shaped the Evolution of Mind. Synthese, 122, 3–28, doi:10.1023/A:1005263825428.
- Wang, F.; Kessels, H.W.; Hu, H. (2014). The mouse that roared: neural mechanisms of social hierarchy. Trends Neurosci., 37, 674–682, doi:10.1016/j.tins.2014.07.005.
- Boer, de, S.F.; Vegt, van der, B.J.; Koolhaas, J.M. (2003). Individual variation in aggression of feral rodent strains: A standard for the genetics of aggression and violence? Behav. Genet., 33, 485–501, doi:10.1023/A:1025766415159.
- Patel, D.; Kas, M.J.; Chattarji, S.; Buwalda, B. (2019). Rodent models of social stress and neuronal plasticity: Relevance to depressive-like disorders. Behav. Brain Res., 369, doi:10.1016/j.bbr.2019.111900.
- Schmidt, M.V.; Scharf, S.H.; Sterlemann, V.; Ganea, K.; Liebl, C.; Holsboer, F.; Mu, M.B. (2010). High susceptibility to chronic social stress is associated with a depression-like phenotype. Psychoneuroendocrinology, 635–643, doi:10.1016/j.psyneuen.2009.10.002.
Related Posts

Using the Observer XT to measure aggressive behavior in Dolphins

Sunshine and romance: ultraviolet light enhances sexual behavior

